Vancouver, B.C. March 31, 2015 – This news release is a corrected version of the news release issued by GLG Life Tech Corporation (“GLG”) on March 31, 2015. The Results from Operations table contained incorrect values for the Gross Profit (Loss) and Loss from Operations line items, and the corresponding percentage of Revenue figures. The corrected figures are presented below. GLG will be filing an Amended & Restated Management Discussion & Analysis on SEDAR as soon as possible. All other information remains the same
GLG Life Tech Corporation (TSX: GLG) (“GLG” or the “Company”), a global leader in the agricultural and commercial development of high-quality zero-calorie natural sweeteners, announces financial results for the three and twelve months ended December 31, 2014. The complete set of financial statements and management discussion and analysis are available on SEDAR and on the Company’s website at www.glglifetech.com.
FINANCIAL HIGHLIGHTS
GLG achieves an 82% fourth quarter sales increase in 2014 and a 25% annual sales growth for 2014 in its stevia business driven by an increase in its international sales. Revenue for the three months ended December 31, 2014, was $7.5 million, an increase of 82% compared to $4.1 million in revenue for the same period last year. Revenue for the year of 2014 was $20.0 million, an increase of 25% compared to $16.0 million in revenue for the prior year. This 25% increase in sales, comparing 2014 to 2013, was driven primarily by an increase of products sold internationally compared to the prior year. International sales were up approximately 75% for the 12 month period ending December 31, 2014, compared to the same period in 2013.
Net Loss from continuing operations before the impact of asset impairment charges for 2014 was $18.8 million compared to $21.7 million in 2013 or a 13% decrease for the year. Net Loss from continuing operations before the impact of asset impairment charges for the fourth quarter of 2014 was $4.6 million compared to $5.9 million in 2013 or a 23% decrease for the quarter. Net Loss from continuing operations for 2014 was $32.6 million compared to $29.8 million in 2013 or a 10% increase for the year. Total asset impairment charges realized for 2014 were $13.8 million compared to $8.1 million in 2013. Loss per share from continuing operations for 2014 was $0.95 per share, compared to $0.89 in 2013, or a 7% increase.
Non-GAAP Financial Measures improved in 2014 for both loss per share from continuing operations and before asset impairment charges and EBITDA margin. Loss per share from continuing operations and before asset impairment charges was $0.55 per share for the year ended December 31, 2014, compared to the prior year ($0.65 per share) or a 15% improvement. Loss per share from continuing operations and before asset impairment charges was $0.13 per share for the fourth quarter of 2014, compared to the same period in 2013 ($0.18 per share) or a 25% improvement. EBITDA margin for 2014 improved to negative 22% from negative 28% in 2013 or a 21% improvement.
CORPORATE AND SALES DEVELOPMENTS
Commencement of Monk Fruit Business
GLG has been active in its plans to become a leading supplier of monk fruit (also known as Luo Han Guo) extract in 2014. In 2014, it undertook a series of significant steps towards that end. In July 2014, these efforts resulted in a milestone contract to supply monk fruit extract to a global leader in the food industry. Some of the significant achievements in GLG’s development of its monk fruit capabilities, including the supply agreement, are described below.
On February 3, 2014, the Company announced that it had filed a patent with the State Intellectual Property Bureau of the People’s Republic of China for its proprietary process for extraction and production of high-purity monk fruit extracts as well as monk fruit formulations used in food and beverage applications. The Company is seeking International Patent Protection under the Patent Cooperation Treaty for this patent. The patent filing has two components; the first addresses GLG’s proprietary industrial scale purification processes for monk fruit and the second addresses monk fruit formulations. Both components leverage our patented and proprietary techniques developed for purification and formulation of high-purity stevia extract products. GLG expects that its proprietary monk fruit technology covered in this patent will result in higher yields of mogroside from the fruit and greater purity of extracts. The company is currently capable of producing monk fruit extracts up to 60% mogroside V purity and is working on 70 to 80% pure mogroside V extracts. These products are expected to be the highest available in the marketplace. Formulations in the patent cover a range of formulations, including stevia monk fruit blends
On July 21, 2014, GLG announced that it had submitted a Generally Recognized as Safe notification with the FDA for its monk fruit extract products. The GRAS notification covers three distinct monk fruit extracts, MV30, MV50, and MV60, each containing a minimum level of mogroside V (30%, 50%, and 60%, respectively). On December 10, 2014, the Company announced that it had received from the FDA a Letter of No Objection regarding the Company’s determination of GRAS status for its monk fruit extracts.
On July 23, 2014, GLG was pleased to announce that it had signed an agreement under which it is producing and supplying monk fruit extract products to one of the global leaders in the food industry. GLG expects revenues from this twelve-month agreement to be approximately $12 million. While initially encompassing a one-year term corresponding to the 2014 growing season, harvest, and subsequent production, GLG and the customer have the express option to extend the agreement over future years. This agreement not only hailed GLG’s entry into the monk fruit production and supply space, it also immediately positioned GLG to be a leading producer of monk fruit products.
In the latter half of 2014, GLG finished establishing its fully integrated supply chain for monk fruit, including obtaining high-quality monk fruit seedlings, contracting with monk fruit growers and storage facilities, and developing its patent-pending processing technology for high-purity monk fruit extract and quality assurance/quality control processes. GLG also completed modifications to its Runhai stevia processing facility to produce the extracts. It also consummated and completed its purchasing of its inaugural monk fruit harvest, purchasing nearly 60 million pieces of monk fruit, while maintaining its commitment to its Fairness to Farmers program. Production of monk fruit extract began in the fourth quarter of 2014, continuing into early 2015. GLG has trademarked its monk fruit products under the trade names MonkGold™ (Mogroside V purities 40% and higher) and MonkSweet™ (Mogroside V purities under 40%).
Producing monk fruit products is a natural extension of GLG’s core stevia product line; these product lines are each naturally sourced sweetener ingredients and monk fruit is often used complementarily. GLG differentiates itself from other monk fruit producers in four ways: (1) its competitive advantage in establishing agriculture systems in China, including the introduction of Good Agriculture Practices (GAP) by its monk fruit farmers, superior monk fruit seedlings and its proven methods to expand the amount of farming in other crops such as stevia; (2) its commitment to its Fairness to Farmers program, whereby it aims to promote a healthy economy via fair, stable income for farmers in the monk fruit growing region; (3) its advanced processing and extraction technology, which will enable GLG to more efficiently and economically produce monk fruit extracts and (4) its large industrial processing capacity, which well positions GLG for anticipated growth in the monk fruit market driven by international food and beverage companies.
Corporate Rebranding
On January 27, 2015, the Company unveiled its new corporate brand and logo, in addition to the launch of its new website (www.glglifetech.com). GLG’s rebranding emphasizes the Company’s Canadian heritage and reflects its new business strategy, which encompasses three complementary product lines. The new website presents a renewed focus on GLG’s closed loop system that includes superior agriculture programs, production excellence, and our focus on sustainability and corporate social responsibility throughout the supply chain.
The vision for the new brand and logo came together in a symbolization of several essential aspects of our Company’s strategy. The maple leaf, a beloved Canadian symbol, forms the centerpiece of our new logo symbolizing our roots as a public company in Canada. 2015 marks GLG’s 10th anniversary as a publically traded company in Canada. The outer portion of the logo – a circular trio of crescents – symbolizes GLG’s three core product lines; stevia extracts, long our flagship product; monk fruit, with GLG entering the market as the highest-capacity producer of this highly desired sweetener; and our Naturals+ line of ingredients that offers both functional ingredients complementary to the sweetener space as well as products tailored to meet particular market needs. The brand and logo well captures the essence of GLG as a proudly-Canadian innovator and leader in the world of natural zero calorie sweeteners.
The launch of GLG’s new website elaborates on these themes, and more. Visitors will find even greater emphasis on our world-class agricultural programs, including the development of superior non-GMO varietals of stevia and, soon, monk fruit, our technological prowess in the production and innovation arena and our commitment to sustainability and corporate social responsibility. Through the vision of its leaders, the excellence of its team members and the holistic nature of and demanding standards manifest throughout its supply chain, GLG leverages these assets to provide leading natural sweeteners and ingredient solutions to businesses globally.
Agricultural Achievements – Super RA Leaf Strain and High-Purity Reb C Strain (“Reb C Gold”)
The last few months have been a resounding success for GLG’s esteemed non-GMO stevia agricultural program. Through our patented and proprietary breeding programs, we have developed two new strains of leaf that are expected to have a significant impact on our ability to offer lower cost products to the global stevia market.
On December 9, 2014, GLG announced the first of two major agricultural breakthroughs, one that could revolutionize the global food and beverage industry’s ability to utilize naturally-sourced Rebaudioside C (“Reb C”). Through GLG’s development of its “Reb C Gold” seedling using its non-GMO patented breeding methodology, the GLG agriculture team developed a new strain containing remarkably high levels of Reb C glycosides. Historically, conventional stevia leaf has had Reb C concentrations of around 1%. GLG’s Reb C Gold strain, however, contains Reb C concentrations verging on 7%. And lab tests show that Reb C comprises 53% of the glycosides in the Reb C Gold leaf, compared to values of 6% to 8% in other strains of stevia leaf – a remarkable 600% increase. What makes GLG’s Reb C Gold seedling even more special is its high Rebaudioside A (“Reb A”) content. Reb C (53%) and Reb A (41%) alone constitute very near 95% of the total steviol glycosides (“TSG”) in the leaf. Reb C and Reb A are two of the besttasting glycosides in the leaf. Moreover, the Reb C Gold strain has TSG levels nearing 13% of leaf content – on the high end for stevia leaf in the market today. Together, these attributes can greatly mitigate the cost and processing challenges previously associated with Reb C. Overall, this achievement is a major leap forward in the natural, non-GMO agronomic development of the historically scarcer steviol glycosides.
On December 15, 2014, GLG announced the second of this year’s major agricultural breakthroughs, having developed a new stevia seedling variety that is expected to decrease the cost of producing highpurity Reb A (or “RA”) stevia extracts by 50% to 60%. Dubbed the “Super RA” variety, this strain contains double the amount of TSG and nearly triple the amount of Reb A glycosides than contained in conventional stevia leaf on the market today. With such a huge increase in Reb A glycoside content, producing one ton of either intermediate or high-purity extract will require far less stevia leaf – the predominant cost factor – than is presently required. Other costs of production will also be reduced correspondingly. In sum, compared to the overall costs of producing Reb A extracts using today’s conventional stevia seedlings, GLG expects costs for the production of Reb A extracts to be cut by more than half.
Furthermore, both of these new strains derive from GLG’s Huinong line of stevia plants. This line, in addition to producing high-Reb A and high-TSG, carries traits of high leaf or biomass yield (typically 30%- 40% bigger than conventional plants) and high disease resistance. GLG has filed for patent protection for each of the two new strains. It expects to implement both the new Super RA and Reb C Gold seedlings in a limited capacity in 2015. GLG looks forward to full-scale commercial implementation of both beginning in 2016.
Product Accomplishments Under FDA’s GRAS Program
Consistent with its role as a leader in the sweetener industry, GLG places great importance on adherence to the Generally Recognized as Safe (“GRAS”) program administered by the United States Food and Drug Administration (“FDA”). Through this program, for each of its core sweetener products, GLG undertakes expert studies and in-depth consultation through GRAS Associates, LLC, which convenes independent panels of scientists to spearhead safety assessments for each product to determine that the product is GRAS. The output of each study is then submitted to the FDA GRAS program, whereupon the submission is reviewed by the FDA. If the FDA finds no issues with the submission, it issues a Letter of No Objection, reflecting the FDA’s view that it has no issue with the Company’s determination that its product is GRAS.
The past year has been a highly productive one for our GRAS submissions. Since the beginning of 2014, we have received the following Letters of No Objection from the FDA:
- On June 3, 2014, the Company announced that it had received a Letter of No Objection regarding its Rebsweet™ and AnySweetPlus™ stevia extract products. These extracts each contain over 95% steviol glycosides (predominantly Rebaudioside A and stevioside), with Rebaudioside A content ranging from 50% to 85%.
- On October 27, 2014, the Company announced that it had received a Letter of No Objection regarding its high-purity Rebaudioside M stevia extract products.
- On December 10, 2014, the Company announced that it had received a Letter of No Objection
regarding its high-purity Monk fruit extract products. This was a significant accomplishment for
the Company’s 2014 entry into the Monk fruit market - On February 17, 2015, the Company announced that it had received a Letter of No Objection regarding its high-purity Rebaudioside C extract products. GLG is the first company to have Rebaudioside C products deemed GRAS in compliance with the FDA’s GRAS program</li >
- Additionally, the Company awaits notification from the FDA regarding its October 2, 2014,
submission to the FDA for its high-purity Rebaudioside D extract products, wherein it notified
the FDA of its determination that the products were GRAS.
GLG has the largest number of stevia extract products certified under the GRAS process, as well as GRAS
status for its Monk fruit extract products. Pursuing and obtaining GRAS designations furthers GLG’s
commitment to maintaining the highest quality standards for its products, and to ensure that each of its
naturally-sourced sweetener products conforms to the GRAS compliance standards.
Launch of organipure™
On March 7, 2014, the Company announced that it was launching its new line of stevia sweeteners— “organipureTM”. The organipureTM line includes purity levels that pair the clean finish and rounded sweetness that GLG stevia extracts are known for with organic certifications that are recognized both in North America and Europe. GLG offers the largest portfolio of stevia extract-based sweetener solutions globally, providing a number of options within the organic line, allowing for the use of organipureTM stevia extract in both high-end and cost-constrained formulations aiming to provide consumers with an organic certified premium finished product. organipureTM was a natural step in the evolution of GLG’s stevia offerings after extensive consultation with its customers and distribution partners. organipureTM represents a premium quality organic product with an exceptional taste profile.
Launch of BevSweet™ and BakeZeroCal™
In February 2015, the Company announced two new products specifically formulated for two industry applications. BakeZeroCal™, for the baking industry, provides significant calorie reduction while also providing the bulking and browning attributes commonly desired by bakers and consumers alike. BevSweet™, for the beverage industry, allows food and beverage companies to reduce calories and naturally sweeten their products with decreased formulation time and with no solubility issues. Each product is a special blend providing an improved taste profile, including a well-rounded sucrose-like sweetness, and ease of use. BakeZeroCal and BevSweet will enable companies to formulate new products and reformulate existing products with less complexity and lower cost.
Appointment of Paul Block to GLG’s Board of Directors
On March 3, 2015, the Company announced the appointment of Mr. Paul R. Block to its Board of Directors. Mr. Block brings a wealth of experience in sales, marketing, and business development as a senior executive in the global food and beverage and sweetener industries. Mr. Block most recently served as Chief Executive Officer of Merisant Worldwide Company, Inc. and the Whole Earth Sweetener Co., LLC. While at Merisant, Mr. Block oversaw the company’s well-recognized line of sweeteners, including the Equal® sweetener brand. Prior to joining Merisant, Mr. Block held C-level positions at Sara Lee Coffee and Tea Consumer Brands, Allied Domecq Spirits USA and Groupe Danone. Mr. Block has been a key figure in developing the global stevia tabletop market through his role as CEO at Merisant and the Whole Earth Sweetener Co., LLC., launching the successful Pure Via® line of tabletop zero calorie stevia sweeteners. Mr. Block is an excellent strategist who will complement our current Board makeup and skill set. He has a proven track record of innovation and building shareholder value in the sweetener and food and beverage industries. Mr. Block’s Board appointment is currently subject to final approval by the TSX.
Reconstitution of Audit Committee
On March 23, 2015, the Company was pleased to appoint Brian Palmieri to the Audit Committee. Mr. Palmieri is currently Vice-Chairman of the Board of Directors. Mr. Palmieri previously served as the Company’s CEO from 2005 until 2008, when he relinquished that role and was named as President and Vice-Chairman. In 2010, Mr. Palmieri relinquished his role as President and has continued to serve the Board in his capacity as Vice-Chairman. Mr. Palmieri is an independent director. Mr. Palmieri replaces David Hall, who joined the Board in 2010, and since that time had been serving on the Audit Committee as Chairman; Mr. Hall continues to serve as a Director on the Board. The Audit Committee now consists of the following three Directors: Madame Sophia Leung (Chairman of the Audit Committee), Madame Liu Yingchun (Member), and Brian Palmieri (Member).
Changes to Convertible Debt Agreement
On September 12, 2014, the Company announced that it had finalized an agreement, then subject to regulatory approval by the TSX, that would result in conversion of a $4.3 million obligation into shares in the Company. The obligation originated with GLG’s disposition of its interest in its prior AN0C joint venture. Pursuant to that disposition, on September 30, 2013, GLG issued a three-year convertible note to China Agricultural Healthy Foods Limited (“CAHFC”) with a principal amount of CAD $4,295,532.65 due September 30, 2016, or convertible into common shares of GLG at $1.80 per share.
The amended agreement required that the principal amount be instead immediately converted into common shares at $1.00 per share, which, as of the September 11th closing price of the Company’s stock, equated to a premium of 435%. The TSX granted approval of the agreement and the amended exercise price. On October 27, 2014, these additional shares issued, and CAHFC become an insider of GLG, holding approximately 11.4% of the issued and outstanding shares of the Company. At the same time, the debt obligation was eliminated.
With this accelerated conversion, GLG removed from its books this substantial $4.2 million liability.
Final Dismissal and Discontinuance of Shareholder Lawsuits
On February 3, 2014, the Company announced that the class action lawsuit filed against GLG for alleged failures to disclose certain information was dismissed. The Company secured a dismissal with prejudice of a securities class action filed against it and two of its officers (CEO Dr. Luke Zhang and President and CFO Brian Meadows) in the United States District Court for the Southern District of New York (the “Court”). In granting the Company’s motion to dismiss the class action, the Court held that the Company had previously disclosed “substantial information [to] the market that suggested precisely that which plaintiffs alleged defendants failed to disclose” under the United States securities laws. The Court further found that “plaintiffs have failed to allege that defendants had a plausible motive to defraud investors,” and noted the fact that Dr. Zhang “purchased a significant number of shares during the putative class period.” Significantly, the Court also ruled that the Company “persuasively argue[d]” that it had also “disclosed all that was required” under Canadian securities regulations. On March 13, 2014, the Company announced that the deadline to appeal the judgment entered in favor of the Company and its officers had passed, and that the dismissal of this class action was final.
In addition, on September 30, 2014, the Company announced that the parallel proposed class action law suits filed in the Supreme Court of British Columbia and in the Ontario Superior Court of Justice have been discontinued. This brings an end to all shareholder actions previously brought against GLG.
OUTLOOK
General Natural Sweetener Market Outlook
The Company continues to see certain big trends driving marketplace. These include health concerns over obesity and diabetes, people looking for food and beverage items with fewer calories, customers favoring natural products and ingredients over artificial ones (such as aspartame), and food and beverage companies continue to be under consumer and even regulatory pressure to provide lowercalorie servings
Monk Fruit Business
GLG officially entered the monk fruit business in 2014, with its announcement of a major supply agreement with a global leader in the food ingredient industry. The Company remains on track for delivering on that agreement. All monk fruit purchased during the Company’s inaugural harvest – nearly 60 million pieces of fruit – has been processed. The Company completed its first shipment in the first quarter of 2015, and expects to deliver the remainder under the agreement by the end of the second quarter. While the initial agreement was for a term of one year, GLG expects to continue to do business with this customer over the coming years, and further expects an increase in product volume to be delivered, starting in Q4 2015.
Overall, the Company has seen much interest in the market for Monk Fruit since the launch of its MonkGold™ extract products. The market reaction has been very positive; more and more companies are exploring the benefits of and need for these extracts, whether as stand-alone sweetener solutions or as used in great-tasting blends with stevia and other ingredients.
Stevia Business
GLG had been predicting a tightening in stevia leaf and product supply, and indeed, this came to pass in late 2014. There had been a decrease in stevia farming, resulting in higher prices for both leaf and, in turn, stevia extract products. We also saw that the surplus stocks that had been available in 2011 and 2012 have largely been depleted and now need to be replenished. As an example, at the end of 2011, GLG had about $100 million in inventory; it now has less than $10 million.
Fortunately for GLG, our agricultural team had a banner year in 2014, with the development of our Super RA and Reb C Gold stevia strains, which, as discussed above, can be used to significantly reduce the amount of leaf required to produce a given amount of stevia extract product. The announcements of these developments attracted significant attention from major food & beverage and food ingredient multi-nationals that have historically been large users of stevia. GLG is working to contract with these customers for one- and two-year supply agreements, who, in light of the stevia shortage, are more willing to enter into such supply agreements. GLG expects to have a number of these complete in the coming weeks, accounting for a significant amount of international business. These commitments will enable GLG to plant with confidence for the 2015 harvest, and also to offer competitive pricing for our stevia extract products.
There has also been continued interest in our blended products that taste better than traditional stevia products alone (such as RA97 or RA95). The extract from our Reb C Gold strain is a great foundation for superior tasting blends. We are also enthusiastic about the potential for our monk fruit / stevia blends.
Naturals+
GLG completed delivery on its first major contracts for its Naturals+ product line in Q1 2015, in line with prior announcements. We continue to see other opportunities for ingredients complementary to the natural high intensity sweetener market from a variety of existing and potential customers, who are interested in these natural ingredients sourced in China. GLG has a dedicated team to help with sourcing these specialized natural ingredients from an extensive and qualified network of suppliers across China. 2015 will be the first year where we see significant revenues coming from this product line.
SELECTED FINANICALS
As noted above, the complete set of financial statements and management discussion and analysis for the year ended December 31, 2014, are available on SEDAR and on the Company’s website at www.glglifetech.com
Results from Operations
The following results from operations have been derived from and should be read in conjunction with the Company’s annual consolidated financial statements for 2013 and 2014.
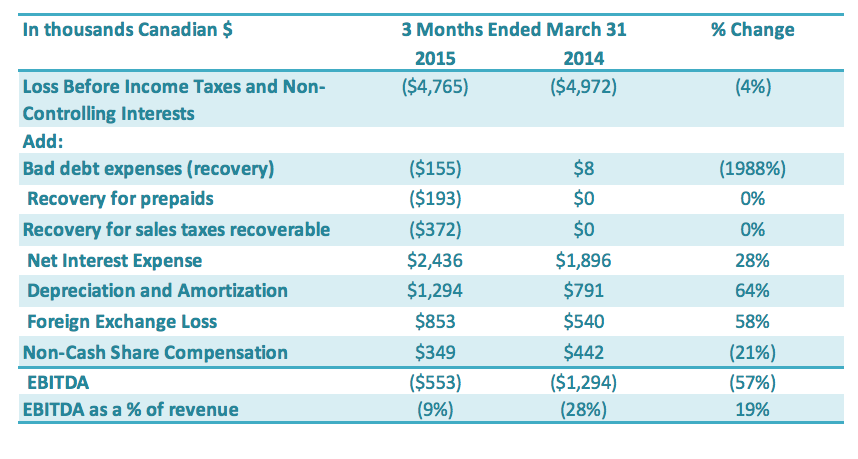
Revenue
Revenue for the three months ended December 31, 2014, was $7.5 million, an increase of 82% compared to $4.1 million in revenue for the same period last year. This 82% increase in revenue, comparing the fourth quarter in 2014 to the fourth quarter in 2013, was due to a 50% increase in sales of products in China relative to the same period in the prior year. We were able to use proceeds of these increased low purity stevia sales to purchase raw material (fruit) for our new product line of monk fruit in the fourth quarter. International sales also increased substantially in the fourth quarter of 2014, relative to the same period last year, increasing by 41%. This reflects the Company’s continuing strategy focusing on increasing its sales of high purity stevia extracts to international customers
Revenue for the year of 2014 was $20.0 million, an increase of 25% compared to $16.0 million in revenue for the last year. This 25% increase in sales, comparing 2014 to 2013, was driven primarily by an increase of products sold internationally compared to the prior year. The main revenue increase came from an increase in international sales, again reflecting the Company’s continuing strategy focusing on increasing its sales of high purity stevia extracts to international customers. International sales were up approximately 75% for the 12 month period ending December 31, 2014, compared to the same period in 2013. International sales have contributed 41.5% of 2014 annual revenues compared to only 29.6% for the same period in 2013.
The other major sales growth initiatives in 2014 include the introduction of the Company’s second zerocalorie natural sweetener—Monk fruit or “Luo Han Guo”—and the sale of other natural ingredients complementary to stevia (our GLG Naturals+ line of products). GLG signed its first contract for the supply of monk fruit in July of 2014; however there was no associated revenue realized in fiscal 2014, as deliveries commenced in the first quarter of 2015. GLG also secured its first sale within the Naturals+ line of products during the second quarter of 2014, with delivery and payments beginning in the fourth quarter of 2014.
Cost of Sales
For the quarter ended December 31, 2014, the cost of sales was $7.3 million compared to $2.3 million in cost of sales for the same period last year ($5.0 million or 222% increase). Cost of sales as a percentage of revenues was 97% compared to 55% in the prior period, an increase of 42 percentage points. The cost of sales as a percentage of revenue was higher for the quarter ended December 31, 2014, compared to prior year due to the impact of a significant increase in stevia raw material costs during the quarter compared to the prior period.
Cost of sales for 2014 was $22.0 million compared to $17.7 million for 2013 or an increase of 4.3 million or 24%. Cost of sales as a percentage of revenues was 110% compared to 111% in 2013, a decrease of 1%. The cost of sales as a percentage of revenue was lower than last year due to the impact of lower idle capacity charges resulting from improved utilization of our facilities due to monk fruit production. Capacity charges charged to the cost of goods sold ordinarily would flow to inventory and is the largest factor on reported gross margin. Only two of GLG’s manufacturing facilities were operating during the year of 2014, and capacity charges of $1.9 million were charged to cost of sales (representing 9% of cost of sales) compared to $2.5 million charged to cost of sales in 2013 (representing 14% of cost of sales).
The key factors that impact stevia cost of sales and gross profit percentages in each period include:
- Capacity utilization of stevia and monk fruit manufacturing plants.
- The price paid for stevia leaf and monk fruit and their respective quality, which are impacted by crop quality for a particular year/period and the price per kilogram for which the stevia and monk fruit extracts are sold. These are the most important factors impacting the gross profit of GLG’s stevia and monk fruit business.
- Other factors which also impact stevia and monk fruit cost of sales to a lesser degree include:
- water and power consumption;
- manufacturing overhead used in the production of stevia and monk fruit extract, including supplies, power and water;
- net VAT paid on export sales;
- exchange rate changes; and
- depreciation.
GLG’s stevia business is affected by seasonality. The harvest of the stevia leaves typically occurs starting at the end of July and continues through the fall of each year. GLG’s operations in China are also impacted by Chinese New Year celebrations, which occur approximately late-January to mid-February each year, and during which many businesses close down operations for approximately two weeks. GLG’s production year runs October 1 through September 30 each year.
Gross Profit (Loss)
Gross profit for the three months ended December 31, 2014, was $0.2 million, a decrease of $1.7 million compared to $1.9 million in gross profit for the comparable period in 2013. The gross profit margin for the three-month period ended December 31, 2014, was 3% compared to 45% for the three months ended December 31, 2013, or a reduction of 42 percentage points from the previous year. The gross margin for the three-month period ended December 31, 2014, was impacted by the product mix of a high volume of low purity stevia extracts for the quarter that had lower gross margin relative to the margins of higher purity stevia extracts. Low purity stevia extract sales increased 99% in the fourth quarter of 2014 compared to the prior period in 2013 and accounted for the majority of the fourth quarter sales for the quarter ended December 31, 2014.
Gross loss for 2014 was $2.0 million, an increase of 16% over $1.7 million in gross loss for the comparable period in 2013. The gross profit margin for the year ended December 31, 2014, was negative 10% compared to negative 11% for the year ended December 31, 2013, or an improvement of 1 percentage points from the previous year. The improvement to gross loss for the year of 2014, was driven by (1) reduced idle capacity charges realized due to the new monk fruit production which commenced in fourth quarter 2014 and (2) the positive change in product mix sold during the year ended December 31, 2014, compared to the prior period with the increased sales to international customers with higher gross profit realized in 2014.
Net Loss Attributable to the Company
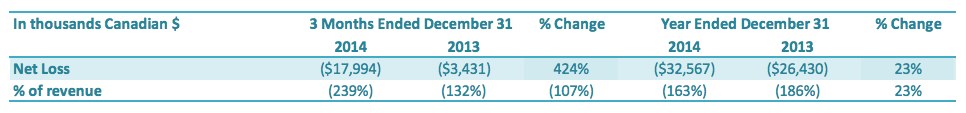
For the three months ended December 31, 2014, the Company had a net loss attributable to the Company of $18.0 million, an increase of $14.5 million or a 424% increase over the comparable period in 2013 ($3.4 million loss). The increase in net loss was driven by (1) an increase in other expenses of $11.0 million, (2) a decrease in gross profit of $1.6 million, (3) a decrease in gain from discontinued operations of $2.0 million offset by an decrease in income tax expense of $0.1 million.
For the year ended December 31, 2014, the Company had a net loss attributable to the Company of $32.6 million, an increase of $6.1 million over the comparable period in 2013 ($26.4 million loss). The increase in net loss was driven by (1) an increase in other expenses of $1.6 million, (2) an increase in gross loss of $0.3 million, (3) an increase in general expenses of $0.9 million and (4) a decrease in gain from discontinued operations of $3.4 million which offset by a decrease in income tax expense of $0.1 million.
Liquidity and Capital Resources
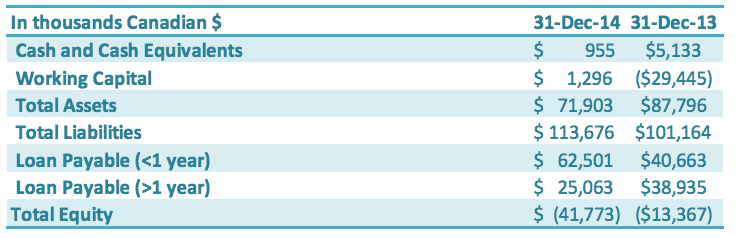
The Company continues to progress with the following measures to manage cash flow of the Company: paying down short-term loans, reducing accounts payable and negotiating with creditors for extended payment terms, working closely with the banks to restructure its loans, arranging financing with its Directors and other related parties, and reducing operating expenditures including general and administrative expenses and production-related expenses. Total loans payable (both short-term and long-term) is $87.6 million as of December 31, 2014, an increase of $7.9 million compared to the previous year ($79.6 million). The increase in loans was primarily driven by working capital required to purchase Monk Fruit against a key customer contract ($7.2 million in loans as of December 31, 2014). The largest impacts on the Company’s increase in negative working capital for the year ended December 31, 2014, capital are (1) the change in classification of $21.0 million long-term debt as of December 31, 2013, to short term debt as of December 31, 2014, and (2) the impairment charges realized on current assets of $7.8 million in the year ended December 31, 2014.
The Company worked with its Chinese banks on restructuring its Chinese debt. By the end of 2014, the Chinese debt with the Agricultural Bank of China had been successfully transferred to China Huarong Asset Management Co., Ltd. (“Huarong”), which is a state-owned capital management company (“SOCMC”). Subsequent to year-end, the Construction Bank of China successfully transferred GLG’s debt to China Cinda Asset Management Co., which is another SOCMC. These two agreements account for approximately 68% of the Company’s outstanding debt with Chinese banks. The nature of the business of these SOCMCs differs from banks, in that they take a long-term outlook on management of debt. For example, instead of simply requiring loan principal and interest payments, the SOCMCs aim to manage debts with greater flexibility, such as long-term loan terms, debt for equity arrangements, flexible debt retirement, and other long-term instruments. This debt is held at the Chinese subsidiary level, and any such potential arrangements would therefore be done at that level rather than at the corporate level. These SOCMCs could also be a source of possible future capital. The Company is still in discussions with these SOCMCs as to final terms – including interest rate and term of the debt – for the transferred debt. Until such terms are confirmed in a formal agreement, the terms of the original loan are represented in the financial statements. The Company further expects to successfully restructure the remaining as yet non-transferred bank debt during 2015.
Selected Annual Information
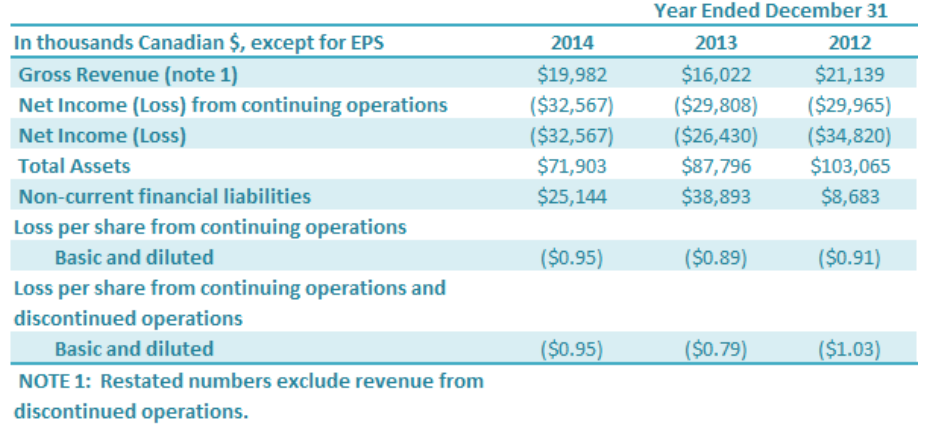
Sales of stevia extracts declined by 24% in 2013 from 2012 and rebounded by 25% in 2014 as the Company increased its sales in the international market and decreased its sales of stevia extracts to other stevia companies primarily located in China. Further the Company commenced its monk fruit operations in the fourth quarter of 2014 and produced its first products under a supply contract announced in July of 2014. The first shipment under this contract was made in the first quarter of 2015 and this new product line will add a new source of sales starting in 2015.
The Company has incurred significant losses for the past three years. In 2012 and 2013, the major drivers of the loss were related to write-downs on stevia inventories due to a supply surplus of stevia extracts in the international marketplace. The Company also had significant operating charges related to its idle facilities during these years. In 2014, the Company commenced operations for its Monk Fruit products. This improved factory utilization starting in the fourth quarter and the first products were manufactured in the fourth quarter of 2014. In 2014, the Company incurred significant impairments related to recoverable sales taxes and prepaid expenses in China ($6.2 million) and Plant, Property and Equipment ($6 million) related to its ion resin equipment; these were offset by reductions in inventory impairments from the prior year ($6.5 million reduction). The market for stevia from the start of the fourth quarter of 2014 has significantly improved since the supply surplus for stevia had been depleted from the market. Market prices for stevia extracts have now increased for the first time in two years. These issues also resulted in a decline in total assets for the years 2012, 2013 and 2014 as inventories were drawn down, and further impairment charges were also taken on obsolete inventory, plant, property and equipment and sales taxes recoverable in China.
During the year ended December 31, 2012 and 2013, non-current financial liabilities increased from $8.7 million in 2012 to $25.4 million by year end 2014. During the year ended December 31, 2013 and 2014, non-current financial liabilities decreased as the Company reclassified $21.0 million long-term bank loan to short-term loan. During these years the Company refinanced short-term bank debt and obtained longer term funding from the Company’s Chairman and CEO and other related parties.
Additional Information
Additional information relating to the Company, including our Annual Information Form, is available on SEDAR (www.sedar.com). Additional information relating to the Company is also available on our website (www.glglifetech.com)
For further information, please contact:
Simon Springett, Investor Relations
Phone: +1 (604) 669-2602 ext. 101
Fax: +1 (604) 662-8858
Email: [email protected]
About GLG Life Tech Corporation
GLG Life Tech Corporation is a global leader in the supply of high-purity zero calorie natural sweeteners including stevia and monk fruit extracts used in food and beverages. GLG’s vertically integrated operations, which incorporate our Fairness to Farmers program and emphasize sustainability throughout, cover each step in the stevia and monk fruit supply chains including non-GMO seed and seedling breeding, natural propagation, growth and harvest, proprietary extraction and refining, marketing and distribution of the finished products. Additionally, to further meet the varied needs of the food and beverage industry, GLG has launched its Naturals+ product line, enabling it to supply a host of complementary ingredients reliably sourced through its supplier network in China. For further information, please visit www.glglifetech.com.
Forward-looking statements: This press release may contain certain information that may constitute “forward-looking statements” and “forward looking information” (collectively, “forward-looking statements”) within the meaning of applicable securities laws. Often, but not always, forward-looking statements can be identified by the use of words such as “plans”, “expects” or “does not expect”, “is expected”, “budget”, “scheduled”, “estimates”, “forecasts”, “intends”, “anticipates” or “does not anticipate”, or “believes” or variations of such words and phrases or words and phrases that state or indicate that certain actions, events or results “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved.
While the Company has based these forward-looking statements on its current expectations about future events, the statements are not guarantees of the Company’s future performance and are subject to risks, uncertainties, assumptions and other factors that could cause actual results to differ materially from future results expressed or implied by such forward-looking statements. Such factors include amongst others the effects of general economic conditions, consumer demand for our products and new orders from our customers and distributors, changing foreign exchange rates and actions by government authorities, uncertainties associated with legal proceedings and negotiations, industry supply levels, competitive pricing pressures and misjudgments in the course of preparing forward-looking statements. Specific reference is made to the risks set forth under the heading “Risk Factors” in the Company’s Annual Information Form for the financial year ended December 31, 2015. In light of these factors, the forwardlooking events discussed in this press release might not occur.
Further, although the Company has attempted to identify factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other factors that cause actions, events or results not to be as anticipated, estimated or intended. The Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
GLG Life Tech Corporation (TSX: GLG) (“GLG” or the “Company”), a global leader in the agricultural and commercial development of high-quality zero-calorie natural sweeteners, is pleased to announce the appointment of Mr. Paul R. Block to its Board of Directors. Mr. Block brings a wealth of experience in sales, marketing, and business development as a senior executive in the global food and beverage and sweetener industries. Mr. Block has most recently served as Chief Executive Officer of Merisant Worldwide Company, Inc. and the Whole Earth Sweetener Co., LLC. While at Merisant, Mr. Block oversaw the company’s well-recognized line of sweeteners, including the Equal® sweetener brand. Prior to joining Merisant, Mr. Block held C-level positions at Sara Lee Coffee and Tea Consumer Brands, Allied Domecq Spirits USA and Groupe Danone. Mr. Block has been a key figure in developing the global stevia tabletop market through his role as CEO at Merisant and the Whole Earth Sweetener Co., LLC., launching the successful Pure Via® line of tabletop zero calorie stevia sweeteners.
Vancouver, B.C. April 27, 2015 – GLG Life Tech Corporation (TSX: GLG) (“GLG” or the “Company”), a global leader in the agricultural and commercial development of high-quality zero-calorie natural sweeteners, is pleased to announce that the United States Food and Drug Administration (“FDA”) has issued a Letter of No Objection for GLG’s high-purity Rebaudioside D (“Reb D”) stevia extract where used as a general purpose sweetener. (Filing No. GRN 548). GLG submitted its application pursuant to the FDA’s GRAS program, through which a company may submit its determination, along with supporting research and studies, that a particular ingredient is Generally Recognized As Safe (“GRAS”). The FDA’s Letter of No Objection confirms that the FDA has no questions regarding GLG’s GRAS notice submitted earlier this year, in which GLG, through in-depth consultation with GRAS Associates, LLC, reported the results of its extensive studies and its conclusion that high-purity Rebaudioside D (> 95% purity) is Generally Recognized as Safe.
High-purity Rebaudioside D stevia extracts have a sweetness level comparable to high-purity Rebaudioside A and have a superior taste profile to Rebaudioside A (less bitterness and astringency). Several major consumer brands have expressed interest in Rebaudioside D. To date, the supply availability and high price of Rebaudioside D extracts have been limiting factors for their broader use in the natural sweetener market. However, GLG is working on an agriculture R&D program to address both of these factors.
GLG is focusing its 2015 agriculture R&D program on improving the amount of Rebaudioside D glycosides through its non-GMO patented and proprietary hybridization process. In its first year of this program, the GLG agriculture team was able to double the percentage of Rebaudioside D in its current variety and plans to continue its efforts to increase the level in 2015. The Company has previously used this patented process to significantly increase the level of Rebaudioside A, Stevioside and most recently Rebaudioside C, and expects to make full use of this process to increase the level of Rebaudioside D glycosides in new varieties of its stevia seedlings. GLG has also patented its proprietary method of extracting Rebaudioside D from stevia leaf
To date, GLG has received nine GRAS Letters of No Objection covering its broad array of high-purity stevia products. No other stevia company can claim such a mark.
For further information, please contact:
Simon Springett, Investor Relations
Phone: +1 (604) 669-2602 ext. 101
Fax: +1 (604) 662-8858
Email: [email protected]
About GLG Life Tech Corporation
GLG Life Tech Corporation is a number one sustainable choice for natural zero calorie sweeteners used by the global food and beverage industry including stevia and monk fruit. GLG’s vertically integrated operations, which incorporate our Fairness to Farmers program and emphasize sustainability throughout, cover each step in the stevia and monk fruit supply chains including non-GMO seed and seedling breeding, natural propagation, growth and harvest, proprietary extraction and refining, marketing and distribution of the finished products. Additionally, to further meet the varied needs of the food and beverage industry, GLG has launched its Naturals+ product line, enabling it to supply a host of complementary ingredients reliably sourced through its supplier network in China. For further information, please visit www.glglifetech.com.
Forward-looking statements: This press release may contain certain information that may constitute “forward-looking statements” and “forward looking information” (collectively, “forward-looking statements”) within the meaning of applicable securities laws. Often, but not always, forward-looking statements can be identified by the use of words such as “plans”, “expects” or “does not expect”, “is expected”, “budget”, “scheduled”, “estimates”, “forecasts”, “intends”, “anticipates” or “does not anticipate”, or “believes” or variations of such words and phrases or words and phrases that state or indicate that certain actions, events or results “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved.
While the Company has based these forward-looking statements on its current expectations about future events, the statements are not guarantees of the Company’s future performance and are subject to risks, uncertainties, assumptions and other factors that could cause actual results to differ materially from future results expressed or implied by such forward-looking statements. Such factors include amongst others the effects of general economic conditions, consumer demand for our products and new orders from our customers and distributors, changing foreign exchange rates and actions by government authorities, uncertainties associated with legal proceedings and negotiations, industry supply levels, competitive pricing pressures and misjudgments in the course of preparing forward-looking statements. Specific reference is made to the risks set forth under the heading “Risk Factors” in the Company’s Annual Information Form for the financial year ended December 31, 2015. In light of these factors, the forwardlooking events discussed in this press release might not occur.
Further, although the Company has attempted to identify factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other factors that cause actions, events or results not to be as anticipated, estimated or intended. The Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.


Leave a Reply